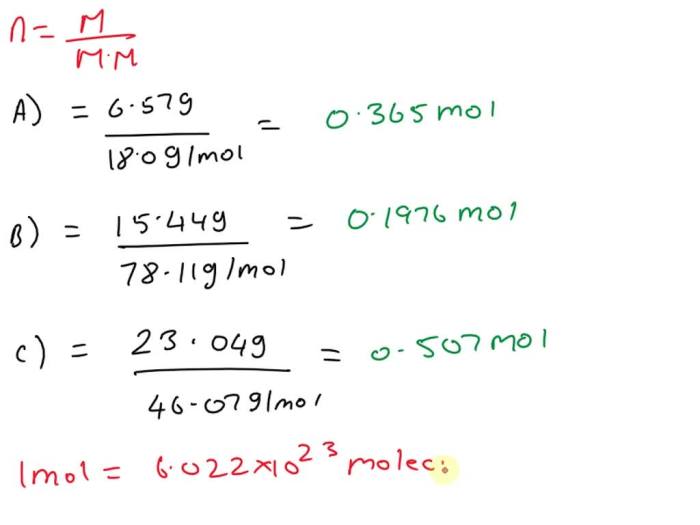Calculate the number of moles of c nc – Calculate the number of moles of C6H6, also known as benzene, is a fundamental concept in chemistry that plays a crucial role in various calculations and applications. This article provides a comprehensive guide to understanding the concept of moles, the steps involved in calculating the number of moles of C6H6, and its significance in chemical reactions and solutions.
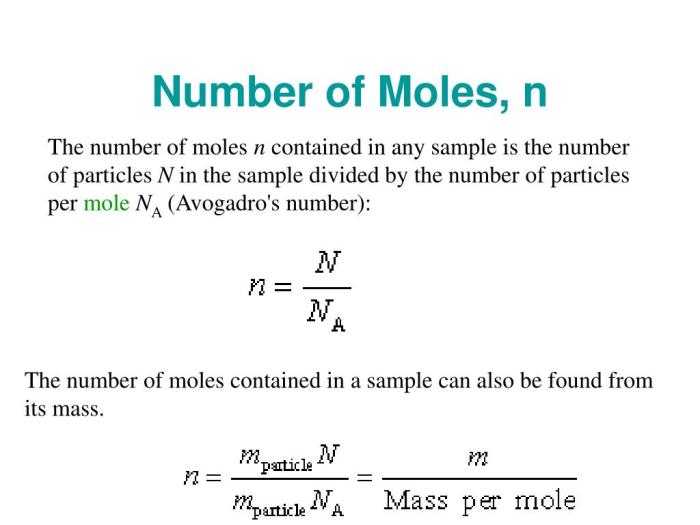
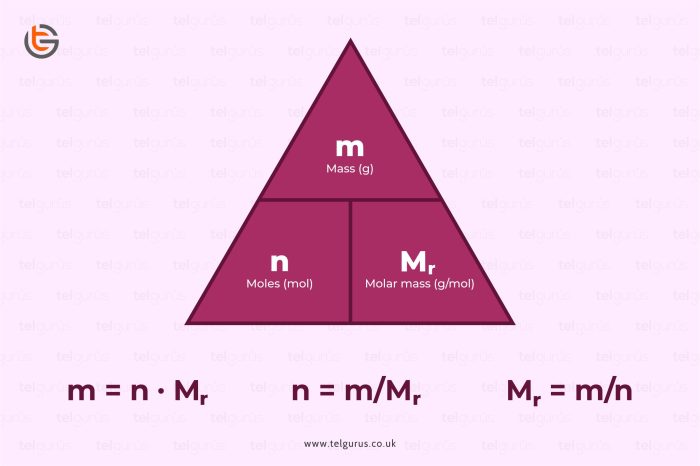
The mole, the SI unit of amount of substance, is defined as the quantity of a substance that contains as many elementary entities as there are atoms in 0.012 kilograms of carbon- 12. The number of moles of a compound can be calculated using the formula: moles = mass / molar mass, where molar mass is the mass of one mole of the compound.
Calculating the Number of Moles of C6H6: Calculate The Number Of Moles Of C Nc
In chemistry, the mole is the SI unit of amount of substance. It is defined as the amount of substance that contains as many elementary entities as there are atoms in 0.012 kilograms of carbon-12.
The mole is a convenient unit for measuring the amount of a substance because it allows us to relate the mass of a substance to the number of atoms or molecules it contains. The molar mass of a substance is the mass of one mole of that substance.
The molar mass of C 6H 6is 78.11 g/mol.
Calculating the Number of Moles of C6H6, Calculate the number of moles of c nc
To calculate the number of moles of C 6H 6in a given sample, we can use the following formula:
moles = mass / molar mass
For example, if we have a sample of C 6H 6that has a mass of 10.0 g, we can calculate the number of moles of C 6H 6in the sample as follows:
moles = 10.0 g / 78.11 g/mol = 0.128 mol
Applications of Calculating Moles
Calculating the number of moles of a substance is an important skill in chemistry. Moles are used in stoichiometry to balance chemical equations. They are also used to determine the concentration of solutions. For example, the molarity of a solution is defined as the number of moles of solute per liter of solution.
User Queries
What is the mole concept?
The mole concept is a unit of measurement used to express the amount of a substance. It is defined as the quantity of a substance that contains as many elementary entities as there are atoms in 0.012 kilograms of carbon-12.
How do I calculate the number of moles of C6H6?
To calculate the number of moles of C6H6, divide the mass of C6H6 by its molar mass (78.11 g/mol). The result will be the number of moles of C6H6 present.
What are the applications of calculating moles?
Calculating moles is essential for various applications in chemistry, including balancing chemical equations, determining the concentration of solutions, and carrying out stoichiometric calculations.