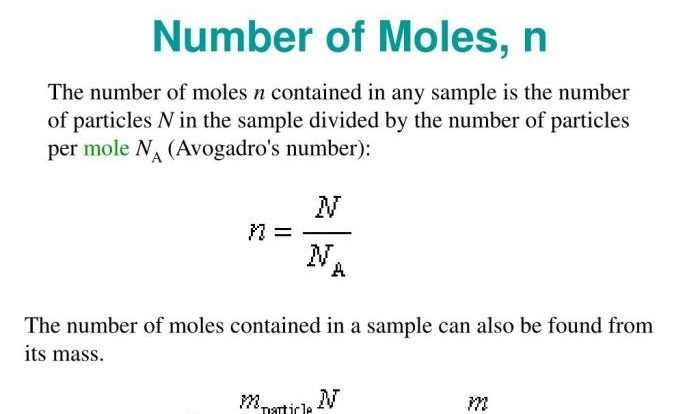
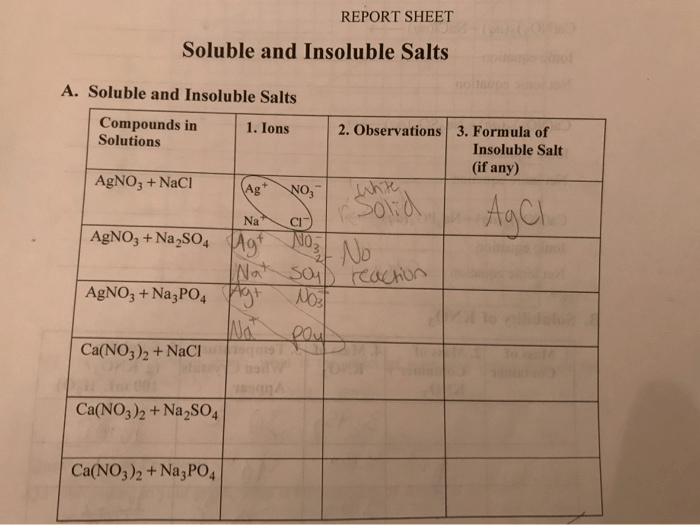Soluble and insoluble salts report sheet – Welcome to the realm of soluble and insoluble salts, where chemistry meets practical applications. Embark on a journey through this report sheet, a repository of knowledge that unveils the intriguing world of salts, their properties, and their diverse uses. Prepare to delve into the depths of solubility, temperature effects, and laboratory experiments, gaining a comprehensive understanding of these fascinating compounds.
Definitions and Classifications
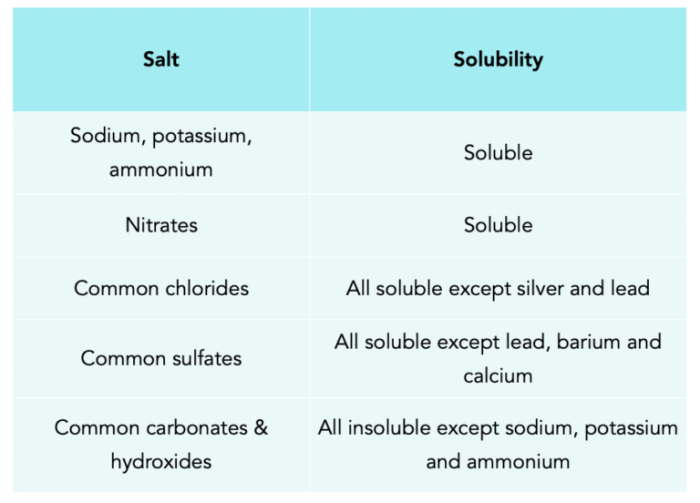
Definitions:
- Soluble salts are ionic compounds that dissolve completely in water, forming a homogeneous solution.
- Insoluble salts are ionic compounds that do not dissolve in water to a significant extent, forming a suspension or precipitate.
Classifications:
Salts can be classified based on their solubility as:
- Highly soluble salts (e.g., NaCl, KCl, Na2SO4)
- Moderately soluble salts (e.g., CaCl2, BaCl2, Pb(NO3)2)
- Slightly soluble salts (e.g., CaCO3, BaSO4, AgCl)
- Insoluble salts (e.g., CaF2, BaSO4, AgI)
Properties of Soluble and Insoluble Salts
Soluble Salts:
- Form homogeneous solutions in water.
- Conduct electricity in aqueous solutions.
- Have high vapor pressures.
- React readily with other substances.
Insoluble Salts:
- Form suspensions or precipitates in water.
- Do not conduct electricity in water.
- Have low vapor pressures.
- Are generally unreactive.
Comparison:
- Soluble salts are more reactive and have higher solubility than insoluble salts.
- Insoluble salts are more stable and have lower solubility than soluble salts.
Solubility and Temperature
The solubility of salts generally increases with increasing temperature.
Soluble Salts:
- As temperature increases, the kinetic energy of water molecules increases, allowing them to break apart more salt crystals.
- This leads to an increase in the number of dissolved salt ions, resulting in higher solubility.
Insoluble Salts:
- The solubility of insoluble salts may also increase slightly with temperature.
- However, this increase is typically negligible compared to soluble salts.
Applications of Soluble and Insoluble Salts, Soluble and insoluble salts report sheet
Soluble Salts:
- Table salt (NaCl): Food seasoning, preservative
- Fertilizers (e.g., NH4NO3, KCl): Agriculture
- Electrolytes (e.g., NaCl, KCl): Biomedical applications
- Water softeners (e.g., Na2CO3): Removing hardness from water
Insoluble Salts:
- Antacids (e.g., CaCO3, Mg(OH)2): Neutralizing stomach acid
- Pigments (e.g., TiO2, ZnO): Paints, cosmetics
- Construction materials (e.g., CaCO3, CaSO4): Cement, plaster
- Abrasives (e.g., SiO2, Al2O3): Sandblasting, grinding
Questions Often Asked: Soluble And Insoluble Salts Report Sheet
What is the key difference between soluble and insoluble salts?
Soluble salts dissolve readily in water, forming homogeneous solutions, while insoluble salts remain suspended in water, forming heterogeneous mixtures.
How does temperature affect the solubility of soluble salts?
Generally, the solubility of soluble salts increases with increasing temperature, as higher temperatures provide more kinetic energy to overcome intermolecular forces.
What are some common applications of soluble salts?
Soluble salts find applications in various industries, including food preservation (e.g., sodium chloride), water treatment (e.g., aluminum sulfate), and medicine (e.g., sodium bicarbonate).